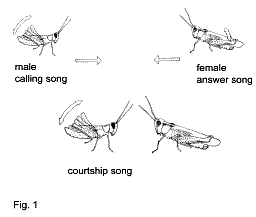
Acoustic communication and song recognition in grasshoppers
Most of the European Orthoptera (katydids, crickets and grasshoppers) actively use
acoustic signals in mating behaviour (Zhantiev, 1980; Popov, 1985; Huber et al., 1989;
Bailey, 1991; Gerhardt, Huber, 2002). In these insects, acoustic communication is the only
way to localize conspecific partner at large distances. Grasshoppers of the subfamily
Gomphocerinae have acquired the most elaborate songs due to the peculiarities of the sound
production apparatus structure and mating strategy (Faber, 1953; Otte, 1970; Helversen,
Helversen, 1983; 1994). A male of Gomphocerinae produces a CALLING song when being alone
(Fig. 1). A female that is ready to mate produces a RESPONSE song. The response of a
female initiates a duet, during which both sexes approach each other. When a male appears
to be nearby a female, the male begins to court. In some species, a COURTSHIP song is
extremely elaborate in terms of a number of different temporal parameters, and sometimes,
conspicuous visual signals are integrated into the courtship (video
clips). Field observations indicate that several competing males often sing around a
female, and courtship songs are very long and frequently repeated. This may facilitate the
female choice and favour competition among males, similar to a "lek"- situation
(Kirkpatrick, Ryan, 1991). 
In Gomphocerinae, the song is produced by stroking a stridulatory file, (pars stridens)
situated on the inside of each hindleg femur, against the radial vein R located on the
ipsilateral wing (Fig. 3b, 4). Usually the sound has a broad frequency spectrum (Fig. 2),
therefore that the specificity of the songs lies not in their frequency band but in the
pattern of amplitude over time. Calling songs in different species of Gomphocerinae reach
a high complexity in their temporal structure (Helversen, Helversen, 1994; Ragge,
Reynolds, 1998; Vedenina, Bukhvalova, 2001; Bukhvalova, 2003; Savitsky, 2004; Vedenina, Helversen, 2009). Using both
hindlegs, the grasshoppers have two separate sound-producing devices that usually work
with a phase shift. The stridulatory movements of the two legs often differ in amplitude
and form, and the legs can exchange roles from time to time (Elsner, 1974). 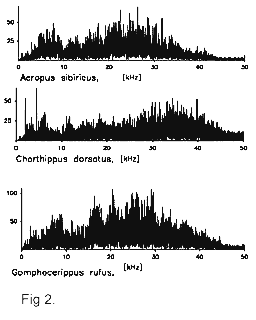
The movements of the hind legs are recorded with an optoelectronic camera made in our
laboratory (IITP RAS) and based on the construction designed by v.Helversen and Elsner
(1977) and developed later by Hedwig (2000). A method of simulataneous recordings of the
leg movements and sound allows us to investigate the connections between the motor and
sound patterns. For example, when the legs are moved in antiphase, the frequency of pulses
is doubled (Fig. 3, Chorthippus albomarginatus). When the legs are moved with a
very slight phase shift in the beginning of a signal, and after that start moving in
antiphase, the distinct pulses disappear, which results in a noise with an amplitude
modulation (Fig. 3, Chorthippus dorsatus). Such masking of distinct pulses caused
by the phase shift in movements of the two legs is widespread in grasshoppers. This shift
varies in different specimens, which results in an increased song intraspecific
variability. Such variability may offer material for sexual selection by the female
choice. 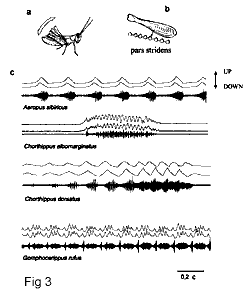
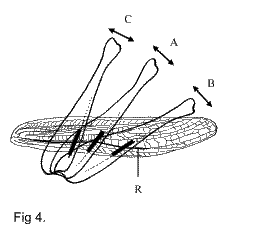
The courtship songs usually contain more elements than the calling songs (Helversen,
1986; Vedenina, Helversen, 2003), which could be partially a result of the functioning of
sound-producing apparatus. Depending on the hind femur position, different parts of the
stridulatory file participate in the sound production. Thus, in Ch. albomarginatus,
the legs held in a low position vibrate slowly (element B in fig. 5), the legs held in a
higher position vibrate more rapidly (element A), and the legs held in an almost vertical
position produce the third, more complex pattern (element C) (Fig. 4). In the courtship
song of Ch. oschei, there are even more elements (Fig. 5). Moreover, in the
beginning of the C element, the legs move into an extra-high position and make a flicking
out movement with the tibiae, which does not produce a sound but may serve as conspicuous
visual display. 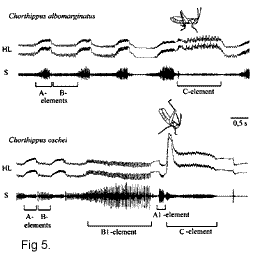
Numerous behavioural and electrophysiological studies conducted during the last three
decades showed that the grasshoppers mainly use temporal parameters (phrase duration, the
number of phrases, syllable/pause ratio, etc.) for the song recognition (Helversen, 1972;
Helversen, Helversen, 1983, 1987, 1994; Stumpner, Helversen, 1992, 1994; Vedenina,
Zhantiev, 1990). In behavioural experiments, the simulated calling songs with distorted
parameters were presented to the females. The frequency of the female response indicated
the attractiveness of the simulated song. Similar experiments with the courtship songs of
grasshoppers were not conducted since the females rarely acoustically answer to the
courting male. The female response can be only evaluated on the basis of acceptance or
refusal of the copulation. 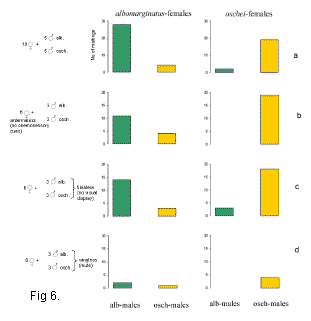
We studied whether the females of two closely related species Chorthippus albomarginatus and Ch. oschei distinguish between con- and heterospecific courtship songs. Although these courtship songs differ in many temporal parameters, the two species are known to hybridize in nature. In choice mating experiments, the females were found to mate rather assortatively with conspecific males, but in 10-20 % of cases they mated with heterospecifics (Fig. 6a). We studied which component of the complex courtship (chemical, visual or acoustic) was the most crucial for conspecific recognition (Fig. 6b-d). The data obtained showed that the acoustic component was the most important for the recognition. The males without wings courted and made copulation attempts as actively as the intact males; however, the percentage of matings was low because the females refused to mate (Fig. 6d).
At present, we continue to study the courtship song parameters that are decisive for the recognition of a conspecific male.